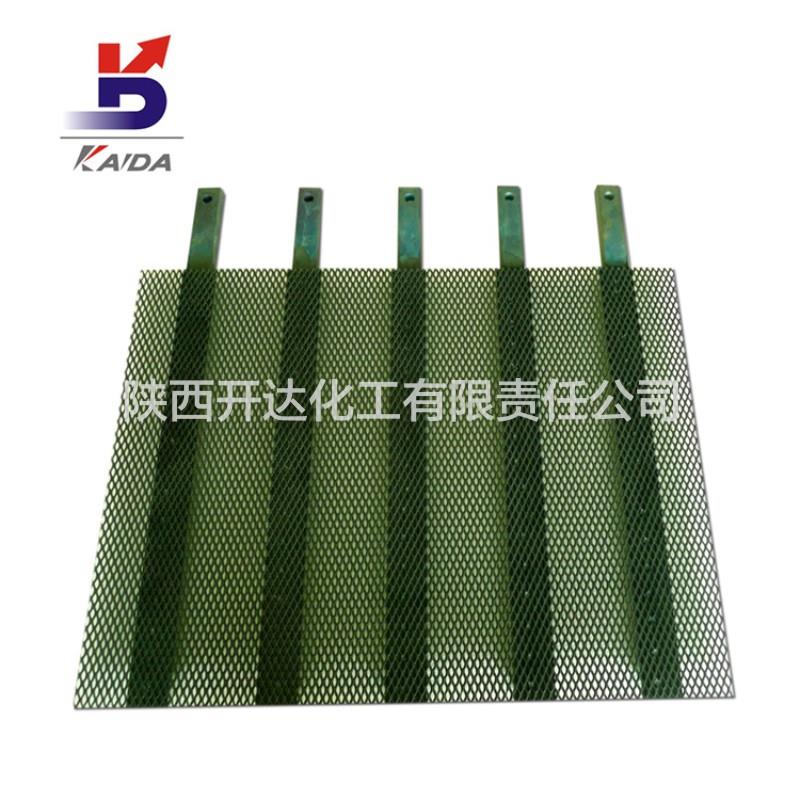
Electrolytic copper foil
Application scope:
Electrolytic copper foil
1. Stable polar spacing
2, can improve the production capacity
3. High carrying current and uniform current distribution
4, no pollution on copper foil
5. Low energy consumption,Long working hours
































 陕公网安备 61030502000254号
陕公网安备 61030502000254号